Publications

59. Arylboration of Cyclic Enones via Cooperative Copper/Palladium Catalysis
Mansour, K.; Saha, P.; Dellermann, L; Newman, S. G. Org. Lett. 2025, 27, 9442. DOI: 10.1021/acs.orglett.5c02841

58. Hydroalkylation of Vinylarenes by Transition-Metal-Free In Situ Generation of Benzylic Nucleophiles Using Tetramethyldisiloxane and Potassium tert-Butoxide
St. Onge, P.; Nugraha, H.; Newman, S. G. Angew. Chem. Int. Ed. 2025, 64, e202421077. DOI: 10.1002/anie.202421077
ChemRxiv: 10.26434/chemrxiv-2023-7k37p

57. 1,5-Diaza-3,7-diphosphacyclooctanes (P2N2): An Underappreciated Ligand Class for Nickel- and Palladium-Catalyzed Heck-Type Cross-Couplings
Isbrandt, E. S.; Newman, S. G. Synlett 2025, 36, 607. DOI: 10.1055/s-0043-1775400

56. Regioselective Synthesis of α-Vinyl Boronates via a Pd-Catalyzed Mizoroki–Heck Reaction
Chen, Z.; Isbrandt, E. S.; Newman, S. G. Org. Lett. 2024, 26, 7723. DOI: 10.1021/acs.orglett.4c02866

55. An SN1-Approach to Cross-Coupling: Deoxygenative Arylation Facilitated by the β-Silicon Effect
Cook, A.; Kassymbek, A.; Vaezghaemi, A.; Barbery, C.; Newman, S. G. J. Am. Chem. Soc. 2024, 146, 19929. DOI: 10.1021/jacs.4c03197
Highlighted in Synfacts
Highlighted in OPRD

54. Understanding and Controlling the Mizoroki-Heck Reaction of Cyclic Enones
Kassymbek, A.; Troyano, F. J. A.; Dimakos, V.; Canterbury, D. P.; Monfette, S.; Roosen, P. C.; Newman, S. G. ACS Catal. 2024, 14, 8193. DOI: 10.1021/acscatal.4c00854
Highlighted in OPRD


53. Synthesis of secondary benzylic alcohols by reductive arylation of aldehydes: α-Phenyl-6-quinolinemethanol.
Thomas, G. T.; Isbrandt, E. S.; Newman, S. G. Org. Synth. 2024, 101, 1. DOI: 10.15227/orgsyn.101.0001
52. Alcohols as substrates in transition metal-catalyzed arylation, alkylation and related reactions
Cook, A.; Newman, S. G. Chem. Rev. 2024, 124, 6078. DOI: 10.1021/acs.chemrev.4c00094
ChemRxiv: 10.26434/chemrxiv-2023-7k37p

51. Controlling Reactivity and Selectivity in the Mizoroki-Heck Reaction: High Throughput Evaluation of 1,5-Diaza-3,7-diphosphacyclooctane Ligands
Isbrandt, E. S.; Chapple, D. E.; Tu, N. P. T.; Dimakos, V.; Beardall, A. M. M.; Boyle, P. D.; Rowley, C.; Blacquiere, J. M.; Newman, S. G. J. Am. Chem. Soc. 2024, 146, 5650. DOI: 10.1021/jacs.3c14612
ChemRxiv: 10.26434/chemrxiv-2023-t9p7j

50. Nickel-Catalyzed Transesterification of Methyl Esters
Zheng, Y.-L.; Daneshfar, O.; Li, J.-Y.; Masson-Makdissi, J.; Pinault-Masson, E.; Newman, S. G. Synlett 2024, 35, 908. DOI: 10.1055/s-0042-1751485
Invited contribution in dedication to Keith Fagnou

48. Deoxygenative Suzuki–Miyaura Arylation of Tertiary Alcohols through Silyl Ethers
Cook, A.; St. Onge, P.; Newman, S. G. Nature Synthesis 2023, 2, 663. DOI: 10.1038/s44160-023-00275-w
ChemRxiv: 10.26434/chemrxiv-2022-f6jvp
Highlighted in Synform

49. Enabling Tools and Techniques for Organic Synthesis: A Practical Guide to Experimentation, Automation, and Computation
Newman, S. G. (ed). Wiley, 2023.













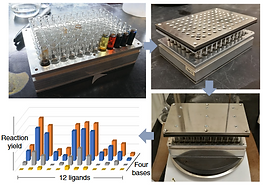










47. Reductive Cleavage of C(sp2)-CF3 bonds in Trifluoromethylpyridines
St. Onge, P.; Khan, S. I.; Cook, A.; Newman, S. G. Org. Lett. 2023, 25, 1030. DOI: 10.1021/acs.orglett.3c00258
Highlighted in OPRD
46. Nickel-catalyzed desulfonylative olefination of β-hydroxysulfones
Cook, A.; Bezaire, M.; Newman, S. G. Org. Chem. Front. 2023, 10, 1399. DOI: 10.1039/D2QO01999J
Invited contribution for the Emerging Investigator Series
45. Evolving progress in ester activation driven by high throughput experimentation
Dimakos, V.; Newman, S. G. in The Power of High-Throughput Experimentation: Case Studies from Drug Discovery, Drug Development, and Catalyst Discovery. Eds. M. Emmert, M. Jouffroy, D. Leitch. ACS Symposium Series 2022.
DOI: 10.1021/bk-2022-1420
44. Esters as Viable Acyl Cross-Coupling Electrophiles
Daneshfar, O.; Newman, S. G. in Amide Bond Activation. M. Szostak, Ed. Wiley 2022.
43. A Morita–Baylis–Hillman Inspired Cross-Coupling Strategy for the Direct α-Arylation of Cyclic Enones
Dimakos, V.; Canterbury, D. P.; Monfette, S.; Roosen, P. C.; Newman, S. G. ACS Catal. 2022, 12, 11557.
42. Reductive 1,2-Arylation of Isatins
Nasim, A.; Thomas, G. T.; Ovens, J. S.; Newman, S. G. Org. Lett. 2022, 24, 7232. DOI: 10.1021/acs.orglett.2c03042
Highlighted in Synfacts
41. Rapid Access to β-Amino Aldehydes by a Ni/Ir Dual-Catalyzed Homologation Reaction
Dimakos, V.; Newman, S. G. Chem Catal. 2021, 1, 1354. DOI: 10.1016/j.checat.2021.11.008
Invited preview
40. Nickel-Catalyzed Reductive Deoxygenation of Diverse C-O Bond-Bearing Functional Groups
Cook, A.; MacLean, H.; St. Onge, P.; Newman, S. G. ACS Catal. 2021, 11, 13337. DOI: 10.1021/acscatal.1c03980
39. Nickel-Catalyzed Aldehyde and Alcohol Arylation Reactions Facilitated by a 1,5-Diaza-3,7-diphosphacyclooctane Ligand
Isbrandt, E. S.; Nasim, A.; Zhao, K.; Newman, S. G. J. Am. Chem. Soc. 2021, 143, 14646. DOI: 10.1021/jacs.1c05661
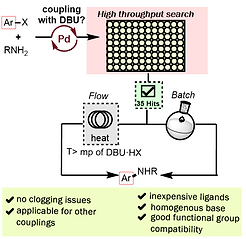
38. Palladium-Catalyzed Cross-Coupling of Superbase-Generated C(sp3) Nucleophiles
Freure, G. P. R.; Skrotzki, E. A.; Lavertu, J.-D. E.; Newman, S. G. ACS Catal. 2021, 11, 12258. DOI: 10.1021/acscatal.1c03180
Highighted as an Editor's Choice article
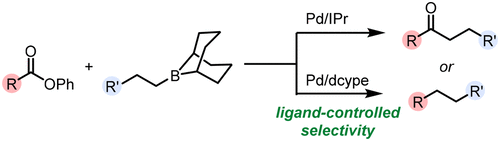
37. Direct Synthesis of Ketones from Methyl Esters by Nickel-Catalyzed Suzuki-Miyaura Coupling
Zheng, Y.-L.; Xie, P.-P.; Daneshfar, O.; Houk, K. N.; Hong, X.; Newman, S. G. Angew. Chem. Int. Ed. 2021, 60, 13476.
36. Ozone-Mediated Amine Oxidation and Beyond: A Solvent Free, Flow-Chemistry Approach
Skrotzki, E. A.; Vandavasi, J. K.; Newman, S. G. J. Org. Chem. 2021, 86, 14168.
DOI: 10.1021/acs.joc.1c00768
Invited contribution for Enabling Techniques for Organic Synthesis special issue
35. Cross-Coupling Reactions with Esters, Aldehydes, and Alcohols
Zheng, Y.-L.; Newman, S. G. Chem. Commun. 2021, 57, 2591.
DOI: 10.1039/D0CC08389E
34. Reaction Screening in Multiwell Plates: High-Throughput Optimization of a Buchwald-Hartwig Amination
Cook, A.; Clément, R.; Newman, S. G. Nature Prot. 2021, 16, 1152.
33. Exhaustive Reduction of Esters Enabled by Nickel Catalysis
Prakash, S.; Cook, A.; Zheng, Y.-Z.; Newman, S. G. J. Am. Chem. Soc. 2020, 142, 8109.
DOI: 10.1021/jacs.0c02405
Highlighted in ChemistryViews
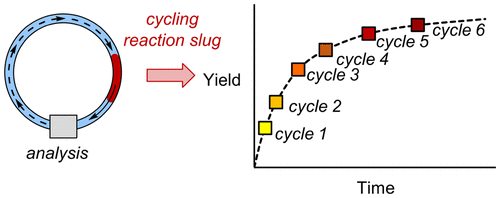
32. Reaction Cycling for Kinetic Analysis in Flow
Sullivan, R. J.; Newman, S. G. J. Org. Chem. 2020, 85, 5464.
Highlighted in OPRD
31. Exploring Homogeneous Conditions for Mild Buchwald-Hartwig Amination in Batch and Flow
Kashani, S. K.; Jessiman, J. E.; Newman, S. G. Org. Process Res. Devel. 2020, 24, 1948.
Invited contribution for Flow Chemistry special issue
30. Nickel-Catalyzed Domino Heck-Type Reactions using Methyl Esters as Cross-Coupling Electrophiles
Zheng, Y.-L.; Newman, S. G. Angew. Chem. Int. Ed. 2019, 58, 18159.
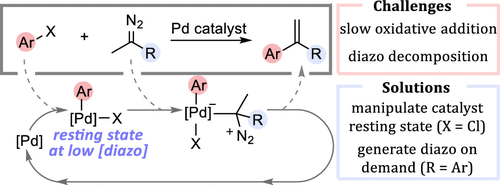
29. Overcoming Scope Limitations in Cross-Coupling of Diazo Nucleophiles by Manipulating Catalyst Speciation and Using Flow Diazo Generation
Sullivan, R. J.; Freure, G. P. R.; Newman, S. G. ACS Catal. 2019, 9, 5263.
28. Ketone Synthesis by a Nickel-Catalyzed Dehydrogenative Cross-Coupling of Primary Alcohols
Verheyen, T.; Turnhout, L. v.; Vandavasi, J. K.; De Borggraeve, W. M.; Isbrandt, E. S.; Newman, S. G. J. Am. Chem. Soc. 2019, 141, 6869.
DOI: 10.1021/jacs.9b03280
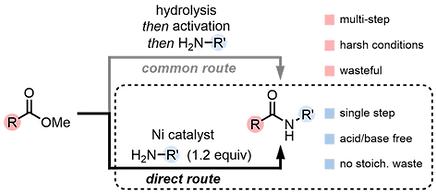
27. Methyl Esters as Cross-Coupling Electrophiles: Direct Synthesis of Amide Bonds
Zheng, Y.-L.; Newman, S. G. ACS Catal. 2019, 9, 4426.
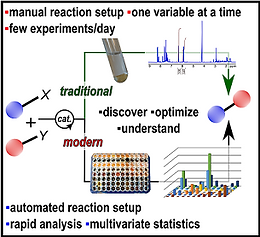
26. High Throughput Strategies for the Discovery and Optimization of Catalytic Reactions
Isbrandt, E. S.; Sullivan, R. J.; Newman, S. G. Angew. Chem. Int. Ed. 2019, 58, 7180.
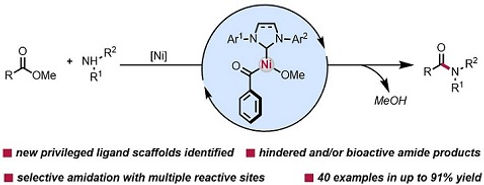
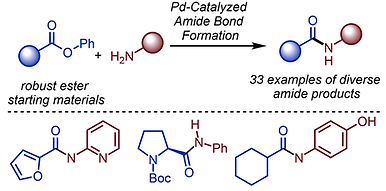
25. Nickel-Catalyzed Amide Bond Formation from Methyl Esters
Ben Halima, T.; Masson-Makdissi, J.; Newman, S. G. Angew. Chem. Int. Ed. 2018, 57, 12925.
24. Flow-Assisted Synthesis of Heterocycles at High Temperatures
Sullivan, J.; Newman, S. in Flow Chemistry for the Synthesis of Heterocycles. K. Sharma & E. Van der Eycken, Eds. Springer 2018.
DOI: 10.1007/7081_2018_18
23. Switchable Selectivity in the Pd-Catalyzed Alkylative Cross-Coupling of Esters
Masson-Makdissi, J.; Vandavasi, J. K.; Newman, S. G. Org. Lett. 2018, 20, 4094.
DOI: 10.1021/acs.orglett.8b01646
22. A High-Throughput Approach to Discovery: Heck-Type Reactivity with Aldehydes
Vandavasi, J. K.; Newman, S. G. Synlett. 2018, 29, 2081.
Invited Synpacts article
Highlighted in OPRD
21. Overcoming Solid Handling Issues in Continuous Flow Substitution Reactions through Ionic Liquid Formation
Kashani, S. K.; Sullivan, R. J.; Andersen, M.; Newman, S. G. Green Chem. 2018, 20, 1748.
DOI: 10.1039/C8GC00618K
20. Chiral Auxiliary Recycling in Continuous Flow: Automated Recovery and Reuse of Oppolzer’s Sultam
Sullivan, R. J.; Newman, S. G. Chem. Sci. 2018, 9, 2130.
DOI: 10.1039/C7SC05192A
Highlighted in OPRD
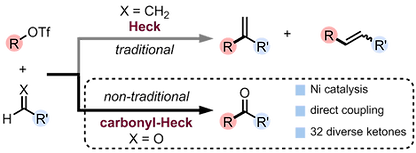
19. A Nickel-Catalyzed Carbonyl-Heck Reaction
Vandavasi, J. K.; Hua, X.; Ben Halima, H.; Newman, S. G. Angew. Chem. Int. Ed. 2017, 56, 15441.
18. Catalytic Deuteration of Aldehydes with D2O
Isbrandt, E. S.; Vandavasi, J. K.; Zhang, W.; Jamshidi, M. P.; Newman, S. G. Synlett 2017, 28, 2851.
Invited contribution in honor of Prof. Snieckus
17. A Cross-Coupling Approach to Amide Bond Formation from Esters
Ben Halima, T.; Vanadavasi, J. K.; Shkoor, M.; Newman, S. G. ACS Catalysis 2017, 7, 2176.
16. Palladium-Catalyzed Suzuki-Miyaura Coupling of Aryl Esters
Ben Halima, T.; Zhang, W.; Yalaoui, I.; Hong, X.; Yang, Y.-F.; Houk, K. N.; Newman, S. G. J. Am. Chem. Soc. 2017, 139, 1311.
DOI: 10.1021/jacs.6b12329
Highlighted in Synfacts
Highlighted in Organic Chemistry Frontiers
15. Inherent Vs Apparent Chemoselectivity in the Kumada-Corriu Cross-Coupling Reaction
Hua, X.; Masson-Makdissi, J.; Sullivan, R. J.; Newman, S. G. Org. Lett. 2016, 18, 5312.
DOI: 10.1021/acs.orglett.6b02631
Highlighted in Synfacts
14. Continuous Thermal Oxidation of Alkenes with Nitrous Oxide in a Packed Bed Reactor
Newman, S. G.; Lee, K.; Cai, J.; Yang, L.; Green, W. H.; Jensen, K. F. Ind. Eng. Chem. Res. 2015, 54, 4166.
DOI: 10.1039/C3GC41942H
13. Pd(0)-Catalyzed Carboiodination: Early Developments and Recent Advances
Petrone, D. A.; Le, C. M.; Newman, S. G.; Lautens, M. RSC Catalysis Series 21. New Trends in Cross-Coupling: Theory and Applications. 2014, 276.
DOI: 10.1039/9781782620259-00276
12. Tools for Chemical Synthesis in Microsystems
Jensen, K. F.; Reizman, B. J.; Newman, S. G. Lab Chip 2014, 14, 3206.
DOI: 10.1039/C3GC41942H
11. Rapid Wolff–Kishner Reductions in a Silicon Carbide Microreactor
Newman, S. G.; Gu, L.; Lesniak, C.; Victor, G.; Meschke, F.; Abahmane, L.; Jensen, K. F. Green Chem. 2014, 16, 176.
DOI: 10.1039/C3GC41942H
10. The Role of Flow in Green Chemistry and Engineering
Newman, S. G.; Jensen, K. F. Green Chem. 2013, 15, 1456.
DOI: 10.1039/C3GC40374B
9. Enantioselective Rh-Catalyzed Domino Transformations of Alkynylcyclohexadienones with Organoboron Reagents
Keilitz, J.; Newman, S. G.; Lautens, M. Enantioselective Rh-Catalyzed Domino Transformations of Alkynylcyclohexadienones with Organoboron Reagents. Org. Lett. 2013, 15, 1148.
DOI: 10.1021/ol400363f
8. Theoretical Study of Pd(0)-Catalyzed Carbohalogenation of Alkenes: Mechanism and Origins of Reactivities and Selectivities in Alkyl Halide Reductive Elimination from Pd(II) Species
Lan, Y.; Liu, P.; Newman, S. G.; Lautens, M.; Houk, K. N. Chem. Sci. 2012, 3, 1987.
DOI: 10.1039/C2SC20103H
7. Palladium-Catalyzed Carbohalogenation: Bromide to Iodide Exchange and Domino Processes
Newman, S. G.; Howell, J. M.; Nicolaus, N.; Lautens, J. Am. Chem. Soc. 2011, 133, 14916.
DOI: 10.1021/ja206099t
6. Palladium-Catalyzed Carboiodination of Alkenes: Carbon-Carbon Bond Formation with Retention of Reactive Functionality
Newman, S. G.; Lautens, M. J. Am. Chem. Soc. 2011, 133, 1778.
DOI: 10.1021/ja110377q
5. The Use of Bromotrichloromethane in Chlorination Reactions
Newman, S. G.; Bryan, C. S.; Perez, D.; Lautens, M. Synthesis 2011, 342.
4. The Role of Reversible Oxidative Addition in Selective Palladium(0)-Catalyzed Intramolecular
Cross-Couplings of Polyhalogenated Substrates: Synthesis of Brominated Indoles
Newman, S. G.; Lautens, M. J. Am. Chem. Soc. 2010, 132, 11416.
DOI: 10.1021/ja1052335
3. Intramolecular Cross-Coupling of gem-Dibromoolefins: a Mild Approach to 2-Bromo Benzofused Heterocycles
Newman, S. G.; Aureggi, V.; Bryan, C. S.; Lautens, M. Chem. Commun. 2009, 5236.
DOI: 10.1039/B912093A
2. Boron-Catalyzed Direct Aldol Reactions of Pyruvic Acids
Lee, D.; Newman, S. G.; Taylor, M.S. Org. Lett. 2009, 11, 5486.
DOI: 10.1021/ol902322r
1. Factors Controlling Extremely Strong AAA-DDD Triply Hydrogen-Bonded Complexes
Newman, S. G.; Taylor, A.; Boyd, R. J. Chem. Phys. Lett. 2008, 450, 210.